

Syn versus anti Biochemistry: Nucleic Acid Chem&Struct

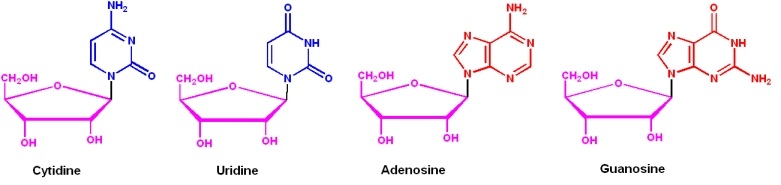
RNA: structure & types Biochemistry: Nucleic Acid Chem&Struct.Nucleic AcidChemistry & Structure Andy HowardIntroductory Biochemistry2 October 2008 Biochemistry: Nucleic Acid Chem&Struct




Syn versus anti Biochemistry: Nucleic Acid Chem&Struct

RNA: structure & types Biochemistry: Nucleic Acid Chem&Struct.Nucleic AcidChemistry & Structure Andy HowardIntroductory Biochemistry2 October 2008 Biochemistry: Nucleic Acid Chem&Struct
